Optimizing the Chilled Ammonia Process for CO2 Capture
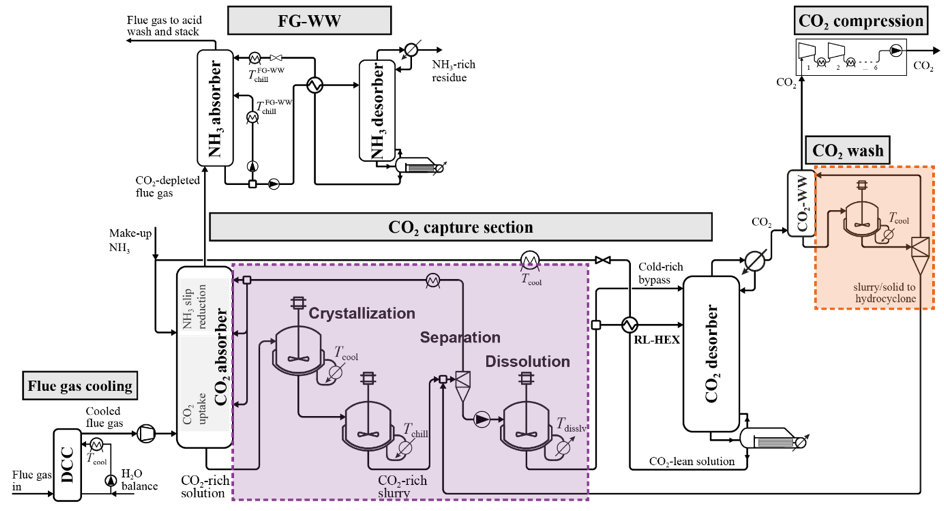
The Chilled Ammonia Process (CAP) is a CO2 capture technology that enables drastic emission reductions from power plants and from CO2- intensive industries. The possible formation of solid NH4HCO3 represents an operational challenge, but also an opportunity for lowering cost and energy penalty. Our project aims at enhancing the performance of the CAP through controlled solid formation.
CO2 capture and storage (CCS) is a bridging technology that enables the drastic and rapid reduction of CO2 emissions as required for the objective of limiting global warming to 2°C (c.f. Paris Agreement). In this chain of technologies, CO2 is captured from large point sources such as CO2 intensive industrial plants (cement, iron and steel, refineries, etc.) as well as coal or natural gas power plants. After separating the CO2 from the flue gas, it is compressed and transported via pipeline to a site where the geological setup allows a safe and durable storage of the CO2 in the deep underground. Geological storage is currently the only technology on a large enough scale, which can guarantee that the massive amounts of captured CO2 do not contribute to climate change.
In the described value chain that has been demonstrated in numerous projects worldwide at various scale, the capture of CO2 is costly and energy intensive step. The Chilled Ammonia Process (CAP) is one of the most promising capture technologies that have been demonstrated at large scale. The CO2 is absorbed in an aqueous ammonia solution and then released in almost pure form upon heating of the solution. The formation of solid ammonium bicarbonate has been identified as a challenge for the stable operation of the CAP due to the potential clogging of process equipment. However, such solid formation also represents an opportunity for lowering cost and energy penalty.
The objectives of our research are threefold:
1. Understanding the thermodynamics of solid formation in the complex CO2-NH3-H2O system (http://www.spl.ethz.ch/research/characterization/cap.html)
2. Development and optimization of a new ammonia-based process that allows exploiting the opportunities related to solid formation (http://www.spl.ethz.ch/research/optimization/cap.html)
3. Application of ammonia-based CO2 capture to cement plants with their extremely high CO2 concentrations of up to 35% (external page https://www.sintef.no/projectweb/cemcap/)
The team involved in this project include Dr. Daniel Sutter, José-Francisco Pérez-Calvo, Federico Milella, Matteo Gazzani, and Prof. Marco Mazzotti, Chair of the ESC and head of the Separation Process Laboratory (SPL).
SPL research deals with adsorption-based separations and chromatography, and with crystallization processes. Applications are in the purification of biopharmaceuticals and in carbon dioxide capture and storage systems.